The lithium-ion battery is a kind of rechargeable battery. Lithium-ion and it’s a kind of battery that is made of lithium metal or lithium alloy as positive/negative material and uses non-aqueous electrolyte solution. It mainly relies on the intercalation and deintercalation of lithium ions between the positive electrode and the negative electrode to achieve energy storage and release.
Analysis of the basic structure of lithium batteries:
The main material composition of lithium batteries: positive electrode material, negative electrode material, electrolyte, separator (separation material)
The simple structure of the lithium battery
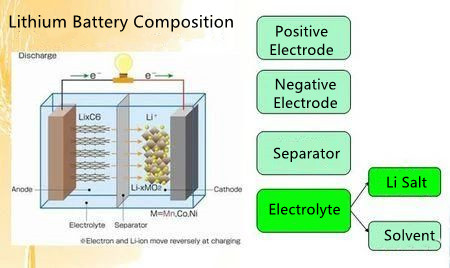
Positive Electrode
From the perspective of battery weight composition, cathode materials account for a large proportion (generally 70% to 80%), because the performance of cathode materials directly affects the performance of lithium-ion batteries, and its cost directly determines the cost of the battery. Cathode materials account for 30% to 40% of the cost of lithium-ion batteries, which also directly affect the energy density and performance of lithium batteries.
Negative Electrode
The anode material is composed of a material with a lower potential relative to the positive electrode, and has a high specific capacity and good charge-discharge reversibility, thus maintaining good dimensional and mechanical stability (without serious deformation) during the process of lithium intercalation. Anode material mainly affects the efficiency of lithium battery, cycle performance, etc., the performance of anode material also affects the performance of lithium battery, anode material accounts for about 10~20% of the total cost of lithium battery. The types of anode materials include carbon-based anodes and non-carbon anodes.
Electrolyte
The electrolyte plays a role of transporting charge between the positive electrode and the negative electrode (similar to the carrier wave in the radio), and has a high ionic conductivity, which should generally reach 1x10-3~2x10-2 S/cm. It affects the energy density, cycle life, power density, safety performance and other factors of lithium battery packs.
Separator
The separator has a fixed pore size and porosity to ensure low resistance and high ionic conductivity, good permeability to lithium ions, good wettability to the electrolyte and sufficient liquid-absorbing and moisturizing ability to maintain ions Conductivity and electronic insulation at the same time, to ensure the mechanical separation of the positive and negative electrodes. In addition, it should have sufficient mechanical properties such as puncture strength and tensile strength, as well as electrolyte corrosion resistance and sufficient electrochemical stability. Power batteries have higher requirements for separators, and composite membranes are usually used.
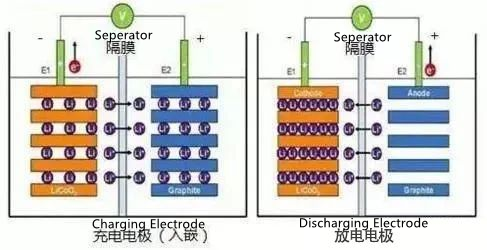
The working principle of lithium battery:
Lithium battery is a kind of rechargeable battery, which mainly relies on the intercalation and deintercalation of lithium ions between the positive electrode and the negative electrode to achieve energy storage and release.
1. Charging process
Driven by the electric field, lithium ions are extracted from the positive electrode lattice, pass through the electrolyte, and are inserted into the negative electrode lattice.
If the voltage is lower than 3V, it must be precharged first. The charging current is 1/10 of the set current. After the voltage rises to 3V, it starts the standard charging. The standard charging process is: constant current charging with the set current, when the battery voltage rises to 4.20V, change to constant voltage charging, keeping the charging voltage at 4.20V. At this time, the charging current gradually decreases, and when the current drops to 1/10 of the set charging current, charging ends. This is a process of general lithium battery charging. If the lithium battery exists in a smart device, its charging mode will be controlled by the smart device software.
2. Discharging process
The discharge process is just the opposite of the charging process. Lithium ions return to the positive electrode under the action of an electric field, and the electrons reach the positive electrode through the external circuit to recombine with the lithium ions. The battery is discharged. At this time, the electron e on the negative electrode runs from the external circuit to the positive electrode, and the positive lithium ion Li+ "jumps" into the electrolyte from the negative electrode. "Climbs" over the crooked hole on the diaphragm and "swims" to the positive electrode, it combines with the electrons that have ran over.
By understanding the charging and discharging process of lithium batteries, we can understand from a microscopic perspective that the capacity of a battery is actually the amount of charge contained in the battery. The greater the current, the faster the discharge rate, and the shorter the battery life.
Why does the battery bulge in normal use or if it is not used for a long time?

There are two phenomena of bulging in the process of charging and discharging:
1. Bulge caused by overcharge
Overcharging will cause all the lithium atoms in the positive electrode material to run into the negative electrode material, causing the original full grid of the positive electrode to deform and collapse. This is also the main reason for the decline in the amount of lithium battery power. In this process, there are more and more lithium ions in the negative electrode, and the excessive accumulation makes the lithium atoms grow out of the tree stump crystals, which makes the battery swell.
2. Bulge caused by over-discharge
During the first charge and discharge of liquid lithium-ion batteries, the electrode material and electrolyte react on the solid-liquid interface to form a passivation layer covering the surface of the electrode material. The formed passivation film can effectively prevent the passage of electrolyte molecules, but Li+ can be freely inserted and extracted through the passivation layer, and has the characteristics of a solid electrolyte. Therefore, this passivation film is called "solid electrolyte interface", referred to as SEI. The SEI film has a protective effect on the negative electrode material, so that the material structure is not easy to collapse, and can increase the cycle life of the electrode material. SEI film is not static, there will be a little change in the process of charging and discharging, mainly part of the organic matter will undergo reversible changes. After the battery is over-discharged, the SEI separator is reversibly damaged, and the SEI that protects the negative electrode material is destroyed, which causes the negative electrode material to collapse, thereby forming a bulging phenomenon.



